I want you to perform a little experiment. Take an egg, put it in a blender, and run it for ten seconds.
Oh, I forgot to tell you to first remove the eggshell. No problem, let’s run the blender in the opposite direction for ten seconds, and we’ll get the egg back.
It doesn’t work, does it? The reason is entropy. The second law of thermodynamics states that the entropy of an isolated system can never decrease. Blending an egg increased its entropy. Unblending it would decrease entropy. But there is a workaround: feed the blended egg to a chicken, and you will get a new egg. Granted, you might have to feed it more than one egg, but still: the miracle of life! Life seems to go against the general trend of the second law of thermodynamics.
Of course, life cannot flourish in a completely isolated system, so the laws of physics are safe. A chicken can produce an egg only by increasing the entropy of its environment and, indirectly, that of the Sun.
Entropy and the Universe
We have some kind of intuitive understanding of entropy as the degree of disorderliness. An egg is highly “ordered,” in that it has an ovoid shell, the white, the yolk and, most importantly, the genetic blueprint for a chicken. It is extremely unlikely that an egg would randomly assemble itself from the primordial soup. And yet, in a way, it did. It took about fourteen billion years, starting from the Big Bang, but it finally arrived to a supermarket near you.
Since entropy has been always copiously produced in the Universe, we are forced to deduce that the initial entropy of the Universe was much lower than it is today. The Universe has been running up the entropy bill at a tremendous pace ever since the Big Bang.
With our simplistic understanding of entropy as the opposite of order, it might be difficult to imagine what it meant for the primordial Universe to be low entropy. Were elementary particles nicely stacked according to their quantum Dewey decimal codes on separate shelves like books in a library? It turns out that, in the presence of gravity, the lowest entropy state is when matter is uniformly distributed throughout the Universe. This might be a little counter-intuitive, considering how blending an egg led to the increase of entropy. But uniform distribution of gravitational mass is a very precarious state. It’s like a needle balanced on its point. At the slightest disturbance, the parts of the volume with infinitesimally higher density will start collapsing on themselves due to gravity. The collapse will be slow in the beginning, but as it keeps increasing local density, it will attract more and more matter resulting in a positive feedback loop.
This is exactly what happened after the Big Bang (as far as we know). Low-entropy uniform soup started slowly curdling to form galaxies and stars. The more non-uniform the distribution of gravitating matter, the higher the entropy.
The ultimate fate of collapsing matter is a gravitational black hole, with all matter concentrated in a singular point. Black holes have extremely high entropy, so much so that it is believed that the current entropy of the Universe is dominated by gigantic black holes in the centers of galaxies.
So why hasn’t the whole Universe collapsed into one gigantic black hole? It’s because the breakneck race toward higher entropy has run against several obstacles. One of them works like a governor in a steam engine. Tiny fluctuations in mass density during the Big Bang were accompanied by tiny fluctuations in velocities of particles. These fluctuations resulted in random distribution of angular momentum. As a result, each collapsing region of the Universe ends up with some randomly assigned net angular momentum. In other words, it spins. And when matter is sucked up towards the center, it starts spinning faster and faster. That’s why every galaxy is spinning. The resulting centrifugal force keeps matter from falling all the way to the center and disappearing into a black hole.
The other obstacle towards reaching maximum entropy is the fact that clumps of matter of certain size turn into stars. When lots of atoms of hydrogen are squished together, they can reach a higher entropy state by fusing into helium. But this process produces excess photons, which keep pushing matter away, thus preventing total collapse. Eventually, the hydrogen burns out, the star undergoes a series of transitions and, depending on its mass, ends up as a supernova, or turns into a brown or white dwarf. What’s left after a supernova explosion can be a neutron star or a black hole.
In a neutron star, further collapse is stalled by another property of matter: Fermi statistics. Neutrons are fermions, and no two fermions may occupy the same quantum state. In particular, you can’t squeeze them all into a very small volume — they repel each other.
Are neutron stars and black holes the end products of the evolution of the Universe? Probably not. There is a strong suspicion that neutrons will eventually decay into leptons — mostly neutrinos, electrons, and positrons. Black holes will evaporate through Hawking radiation. The Universe will eventually reach its thermal death: an ever expanding volume filled with photons and leptons.
What’s Life Got to Do with It?
So far we’ve seen that matter has properties that tend to slow down the ratchet of entropy. Our Sun, for instance, could increase its entropy tremendously by turning all its hydrogen into helium in one fell swoop while collapsing to form a black hole. It can’t do that because of the heat and radiation pressure generated in the process. And even if all the heat were siphoned out, the leftover neutrons would congeal into a solid neutron star, preventing further collapse.
So the Sun is doing its best, under the circumstances, trying to dissipate the excess of energy. It does it mostly by radiating high energy photons. These are the photons of visible and ultraviolet light that warm up the Earth. The Earth, in turn, re-radiates this heat in the form of low energy infrared photons.
It turns out that turning high energy photons to low energy photons increases overall entropy. So, in its small way, the Earth speeds up the rise of entropy. In fact, it does it better than, for instance, Mercury; because the Earth has the atmosphere and the oceans, which are good heat sinks, and because it spins on its axis, transporting the accumulated heat from the sunny side to the shaded side, where it’s radiated into space in the form of infrared photons.
But Earth has another secret weapon that speeds up the advent of the heat death of the Universe: life. To begin with, living organisms consume energy during the day. They also need energy to survive at night, so they came up with clever ways to store energy in chemical compounds. They can then cash their savings at night, all the while radiating heat. At higher latitudes, they also store energy during summer and expend it during winter.
A steppe is better at entropy production than barren land; a forest or a jungle is still better. But human civilization is the best. Our cars, factories, cities, air conditioners, all produce entropy at a much faster pace than bare nature. We’re good at it!
The Self-Organizing Principle
The advent of life on Earth is often attributed to something called the self-organizing principle. It’s just a name for what happens in systems that are away from thermodynamic equilibrium. In those systems it is often possible to speed up the increase of entropy by organizing things a little better.
The simplest example of this is when you heat a layer of liquid in a pan. The liquid can transport energy by thermal conduction, which leads to overall raise in entropy. But there is a faster way: the heated liquid at the bottom of the pan is lighter than the cooler liquid at the top, so it can float to the top. The heavier liquid at the top can then sink to the bottom. This is called convection, and it’s faster than conduction. The only problem is that the two streams of liquid have to negotiate the flow, because they can’t both pass through the same point simultaneously. In fact, in the ideal case, they would be deadlocked. What happens in reality is an amazing feat of self-organization: regularly spaced hexagonal convection cells called Bénard cells emerge in the heated liquid.
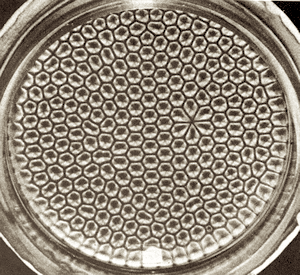
A honeycomb pattern of Bénard cells suggests that order may be spontaneously generated in situations when it can speed up the production of entropy. If you have rich enough environment and wait long enough, more and more complex patterns that ease the production of entropy may emerge — such as life itself.
But life doesn’t emerge everywhere. As far as we know there’s no life on the Moon and no (visible) life on Mars. What’s different about Earth is that it is, and has always been, very turbulent. For starters, we have water that is constantly changing state. It’s boiling in hydrothermal vents, liquid in the oceans, solid in the ice caps; it’s precipitating from the atmosphere and evaporating from pools. It dissolves lots of chemical compounds and makes colloids with others. Continental plates keep shifting resulting in constant volcanic activity. New minerals are brought up from the depths and exposed to erosion. We also have a large Moon that’s causing regular tides, and the Earth’s axis of rotation is tilted resulting in changing seasons. On top of that, we have occasional comets causing impact winters. We can’t complain about lack of entertainment on Earth.
Here’s what I think: Life can only emerge and thrive on the edge. Our planet has been on the edge for a few billion years. Conditions on Earth have always been barely short of wiping the life out and, paradoxically, this is exactly what makes life possible. The Earth is a living proof that what doesn’t kill you, makes you stronger. There have been uncountable attempts on the life on Earth and they all resulted in accelerating the evolution towards higher life forms. I know that it might be controversial to call one form of life higher than another, but there is an objective measure that we can use, and that’s the efficiency of turning energy into entropy. In this respect, humans are indeed the highest form of life. We were able to tap into sources of energy that have been forgotten by nature for hundreds of millions of years in the form of coal, oil, and gas. We use all this to speed up the increase of entropy.
Why Are We Alone?
You might be familiar with the Fermi paradox. In essence, the question is: if life is inevitable, why haven’t we seen it all over the Universe. And judging by how quickly life emerged on Earth– essentially as soon as the water condensed into oceans– life seems to be inevitable, at least on Earth-like planets, which are very common in the Universe. And life — civilized life in particular — being so good at producing vast amounts of entropy, should eventually make itself conspicuous on the cosmic scale.
On the other hand, we don’t know how many planets are “on the edge,” and how narrow the edge is. It’s possible that for an Earth-like planet to enter the life-creating period is a relatively common occurrence — possibly right after the water gathers into oceans. Finding remnants of life on Mars would give support to this idea. But the Earth has been walking this narrow path between stagnation and destruction for more than four billion years. There have been long periods of stagnation: there was the snowball Earth when the oceans froze over, and the “boring billion,” when the air was filled with the smell of rotten eggs. There have been major extinction events, like the asteroid impact that wiped out the dinosaurs.
Being on edge means that you are likely to fall off. You either die of boredom (that’s what might have happened on Mars), or you get wiped out by a cataclysm (if the Chicxulub asteroid were a tad larger, the Earth could have been sterilized). It might be extremely unlikely to stay for a few billion years on the narrow path that leads from Bénard cells to a space-faring civilization. We might actually be the first to reach this level in our cosmic neighborhood. Life on Earth could be more like a professional Russian-roulette player than a nine-to-five worker.
There is also something we don’t quite get about cosmic timescales. For the last few hundred of years the powers of humanity have been growing exponentially. From the cosmic perspective, humanity looks like a sudden bloom that took over a stagnant pool on the outskirts of the Galaxy. We foolishly imagine that we can sustain this level of progress and in short time colonize the Solar system and reach for the stars. But one thing we know for sure about exponential growth is that it’s not sustainable in the long run. We are not only going to bump our heads against unbreakable laws of physics, but we’ll also have to deal with the limitations of human mind. And all other civilizations that might be out there will have to deal with the same problems. This might explain why we are not seeing them.
In fact, we could reverse this reasoning and argue that the fact that we don’t detect any signs of alien civilizations suggests that the obstacles that we see in front of us are not easily overcome. In particular:
- The speed of light limits our ability to travel and exchange information at large distances. This is one of the hardest limits, because special relativity is the foundation of all physics.
- The coupling of gravity to other forces is extremely weak, so the prospects of controlling gravity and counter-balancing acceleration are virtually non-existent. This means that there is no easy way to shrink the enormous distances between stars — no warp drive.
- The size of the atom and the speed of light limit our ability to store and process information. This prevents us from extending the capabilities of our brains to discover and explore the laws of the Universe.
These three limits can also be related to three fundamental theories: special relativity, general relativity, and quantum mechanics, respectively.
So what does the future have in stock for humanity? It looks like we are reaching the end of exponential expansion. There hasn’t been any major breakthrough in fundamental physics for almost half a century, we are seeing the tail end of the Moore’s law, and the population of Earth is finally stabilizing. If we don’t wipe ourselves out from the face of the Earth, we might be facing a boring millennium, if not a boring million. And it’s entirely possible that we are surrounded by other civilizations that have already entered their boring periods. If they eventually graduate to the next stage, they will be ready to help the Universe increase its entropy on a vastly larger scale. Hopefully humanity will still be around to see the Galaxy blooming with sentient activity.
March 28, 2018 at 2:12 pm
Yes, all the interesting stuff happens “on the edge” between the simple order of the original singularity and simple coldness of the eventual heat-death – like the boundary of a Julia set.
This seems related to a sort of Kolmogorov complexity – describing the t=0 and t=end of the universe seems rather simple (almost read best as scripture), while describing the current state of affairs requires describing all environments, the DNA/RNA of all life, and the artifacts of life such as garbage dumps and the complete works of Shakespeare. (Whether completely describing this “current state” is sufficient to predict future states given QCD and “free will” is yet a whole other bag of worms.)
Another analogy is art and other human endeavors, where one must have sufficient resources, but also suffer sufficient constraints to produce something of value.
March 28, 2018 at 2:34 pm
Hi Bartosz,
Ohhh amazing post!!
Thanks for sharing your knowledge.
March 29, 2018 at 2:40 am
Im am also thankful for sharing your knowledge 🙂
March 29, 2018 at 3:23 am
It soon may happen that blockchain miners will be one of the highest form of life on Earth.
April 7, 2018 at 12:46 pm
Beautifully written.
May 9, 2018 at 12:34 am
If the time fluctuated back and forth, it could either:
1. roll back back perfectly, unmixing eggs and shells and also erasing any memory of mixing those, then start going forth again (perhaps exploring another path then the one “before”).
2. fluctuate on the way back, reaching a different state than what the original starting point was, what would kind of make such going back to be just another way of going forth.
In both cases I think an observer that takes part in this could only see the entropy developing.
Exponential phase of a growth in nature usually ends due to resource depletion.
The humanity logical successor, AI, has resources for a decent exponential growth in the asteroid belt.